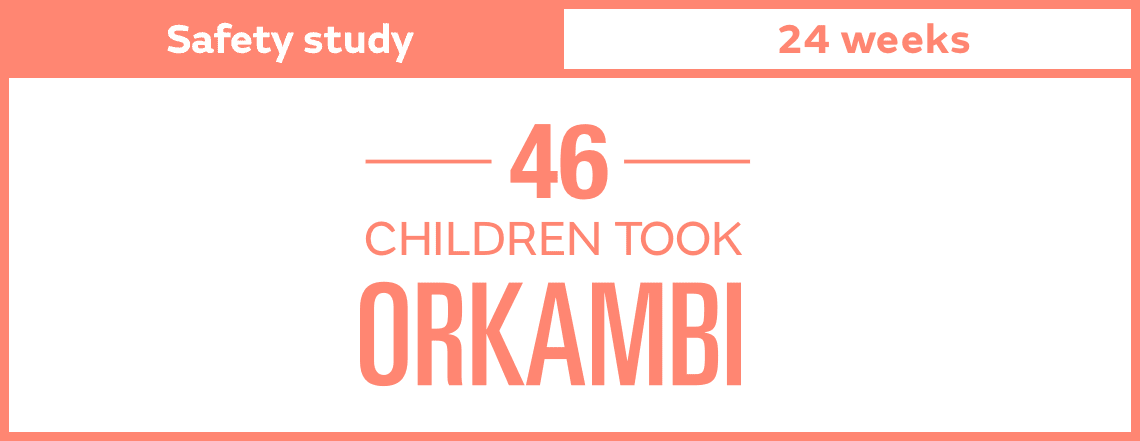
Keep in mind, results shown are an average for all people studied and differed among individuals. You or your loved one may have a different experience.
Measuring the results of treatment
After beginning treatment, you or your loved one may not always feel a difference on a day-to-day or even week-to-week basis. That’s why it’s important to know what to look for and to discuss your results with your doctor. You may want to review certain measures with your doctor to compare them with how they were when treatment was first started.
IMPORTANT SAFETY INFORMATION
Before taking ORKAMBI, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems
- are allergic to ORKAMBI or any ingredients in ORKAMBI. See the Patient Information for a list of ingredients
- have kidney problems
- have lung problems
- have had an organ transplant
- are using birth control (hormonal contraceptives, including oral, injectable, transdermal, or implantable forms). Hormonal contraceptives should not be used as a method of birth control when taking ORKAMBI. Talk to your doctor about the best birth control method you should use while taking ORKAMBI
- are pregnant or plan to become pregnant. It is not known if ORKAMBI will harm your unborn baby. You and your doctor should decide if you will take ORKAMBI while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if ORKAMBI passes into your breast milk. You and your doctor should decide if you will take ORKAMBI while you are breastfeeding
What is ORKAMBI® (lumacaftor/ivacaftor)?
What is ORKAMBI®
(lumacaftor/ivacaftor)?
ORKAMBI is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients aged 1 year and older who have two copies of the F508del mutation (F508del/F508del) in their CFTR gene.
ORKAMBI should not be used in patients other than those who have two copies of the F508del mutation in their CFTR gene.
It is not known if ORKAMBI is safe and effective in children under 1 year of age.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
ORKAMBI may affect the way other medicines work, and other medicines may affect how ORKAMBI works. The dose of ORKAMBI may need to be adjusted when taken with certain medicines. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Especially tell your doctor if you take:
- antibiotics: rifampin (RIFAMATE®, RIFATER®) or rifabutin (MYCOBUTIN®)
- seizure medicines: phenobarbital, carbamazepine (TEGRETOL®, CARBATROL®, and EQUETRO®), or phenytoin (DILANTIN®, PHENYTEK®)
- sedatives and anti-anxiety medicines: triazolam (HALCION®) or midazolam (DORMICUM®, HYPNOVEL®, and VERSED®)
- immunosuppressant medicines: cyclosporine, everolimus (ZORTRESS®), sirolimus (RAPAMUNE®), or tacrolimus (ASTAGRAF XL®, ENVARSUS XR®, PROGRAF®, and PROTOPIC®)
- St. John’s wort
- antifungal medicines including ketoconazole, itraconazole (such as SPORANOX®), posaconazole (such as NOXAFIL®), or voriconazole (such as VFEND®)
- antibiotics including telithromycin, clarithromycin (such as BIAXIN®), or erythromycin (such as ERY-TAB®)
What should I avoid while taking ORKAMBI?
- Do not eat or drink grapefruit products during your first week of treatment with ORKAMBI. Eating or drinking grapefruit products can increase the amount of ORKAMBI in your blood
What are the possible side effects of ORKAMBI?
ORKAMBI can cause serious side effects, including:
- Worsening of liver function in people with severe liver disease. The worsening of liver function can be serious or cause death. Talk to your doctor if you have been told you have liver disease as your doctor may need to adjust the dose of ORKAMBI
- High liver enzymes in the blood, which can be a sign of liver injury in people receiving ORKAMBI. Your doctor will do blood tests to check your liver:
- before you start ORKAMBI
- every 3 months during your first year of taking ORKAMBI
- every year while you are taking ORKAMBI
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- yellowing of your skin or the white part of your eyes
- loss of appetite
- nausea or vomiting
- dark, amber-colored urine
- confusion
- Serious allergic reactions have happened to people who are treated with ORKAMBI. Call your doctor or go to the emergency room right away if you have any symptoms of an allergic reaction. Symptoms of an allergic reaction may include:
- rash or hives
- tightness of the chest or throat or difficulty breathing
- swelling of the face, lips, and/or tongue, or difficulty swallowing
- light-headedness or dizziness
- Breathing problems such as trouble breathing, shortness of breath or chest tightness in patients when starting ORKAMBI, especially in patients who have poor lung function. Call your doctor right away if you experience these symptoms
- An increase in blood pressure in some people receiving ORKAMBI. Call your doctor right away if you have an increase in blood pressure
- Abnormality of the eye lens (cataract) in some children and adolescents receiving ORKAMBI. If you are a child or adolescent, your doctor should perform eye examinations before and during treatment with ORKAMBI to look for cataracts
The most common side effects of ORKAMBI include:
- breathing problems such as shortness of breath and chest tightness
- nausea
- diarrhea
- fatigue
- increase in a certain blood enzyme called creatine phosphokinase
- rash
- gas
- common cold, including sore throat, stuffy or runny nose
- flu or flu-like symptoms
- irregular, missed, or abnormal periods (menses) and increase in the amount of menstrual bleeding
Side effects seen in children are similar to those seen in adults and adolescents. Additional common side effects seen in children include:
- cough with sputum
- stuffy nose
- headache
- stomach pain
- increase in sputum
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ORKAMBI. Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
For further information, please see full Prescribing Information, including Patient Information.